Deuteration of arenes in pharmaceuticals via photoinduced solvated electrons
By:Yi Tao;Cuihua Jin;Chuanwang Liu;Jiawei Bu; Ling Yue; Xipan Li;Kangjiang Liang;Chengfeng Xia
Chem
DOI:https://doi.org/10.1016/.chempr.2024.06.029
Published:2024-07-18
Abstract
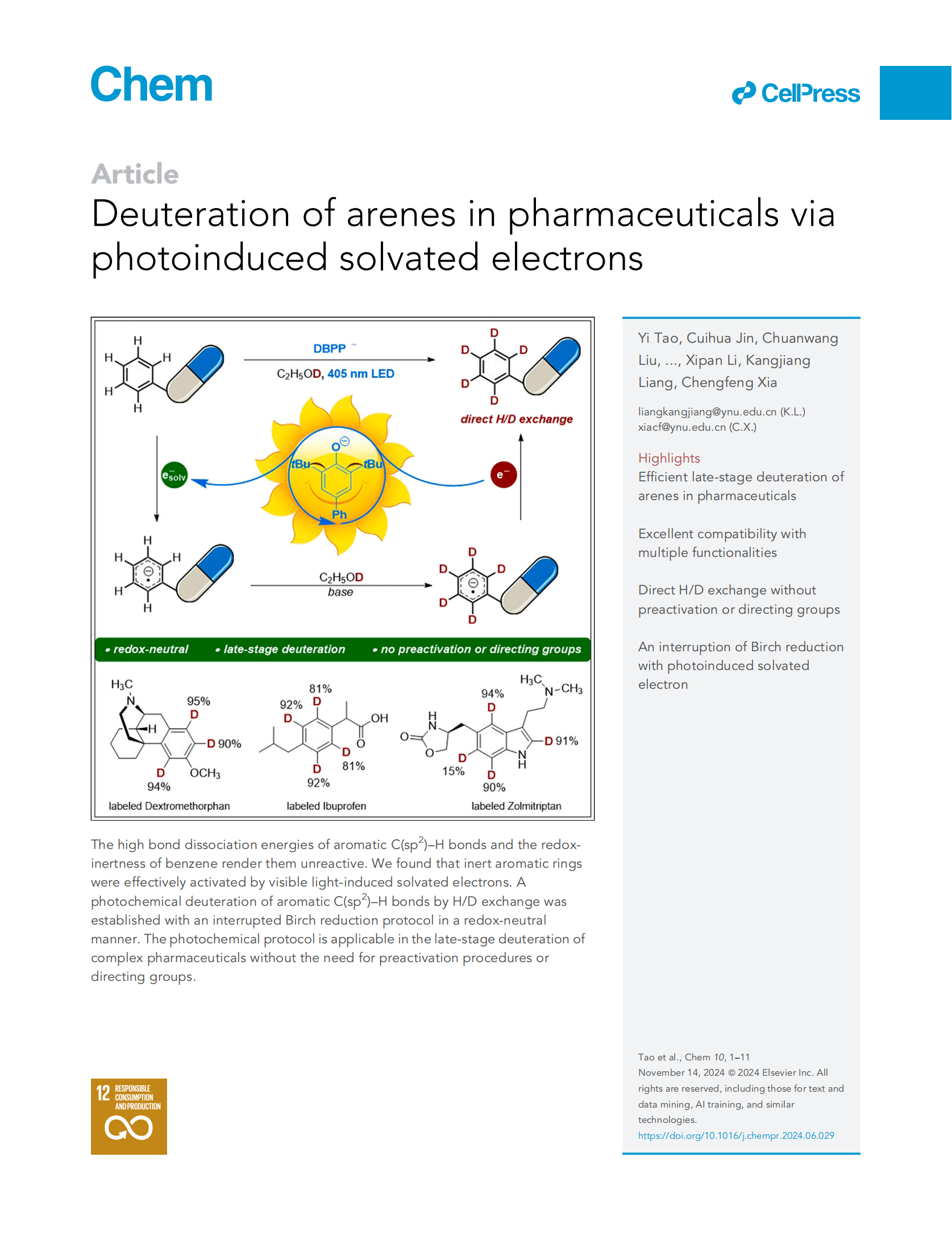
Deuterium incorporation into pharmaceutical molecules has been recognized as having a positive impact on drug efficacy and safety,allowing improvements in pharmacokinetic and/or toxicity profiles.Due to the high chemical inertness of arenes toward the hydrogen atom and the electron transfer processes, the visible light-induced direct deuteration of aromatic C(sp2)–H bonds via hydrogen is otope exchange remains unexplored. Herein, we report a photochemical deuteration protocol for efficient incorporation of deuterium into arenes in a single step, tolerating manifold functionalities in pharmaceutical compounds. Mechanistic studies provided evidence that solvated electrons were generated by light illumination with a phenolate-type photocatalyst and were involved in deuterium incorporation. This protocol was successfully applied to the late-stage deuteration of pharmaceuticals by photochemical aromatic H/D exchange on arenes.