Recently, the Aqueous Battery Innovation Team from the College of Sciences of NEU made a significant advancement in zinc-iodine battery research. Their paper, Diatomic Catalysts for Aqueous Zinc-Iodine Batteries: Mechanistic Insights and Design Strategies, was published in the prestigious Angew. Chem. Int. Ed. journal. Hei Peng, a PhD student from the College of Sciences, is the lead author, with Associate Professor Song Yu from the Department of Chemistry serving as the corresponding author. NEU is the primary institution.

Aqueous Zinc-Iodine Batteries (AZIBs) are known for their high safety, low cost, and no pollution, and expected to be a good choice for next-generation smart grid energy storage system. However, these batteries face challenges such as sluggish redox kinetics, significant self-discharge, limited lifespan, and low Coulombic efficiency, which hinder their broader application. To address these issues, researchers have focused on the physical and chemical confinement effects of iodine species, aiming to accelerate the conversion from triiodide (I₃⁻) to iodide (I⁻) and mitigate the shuttle effect. Single-atom catalysts (SACs) have garnered attention in this field due to their near-100% atomic utilization, high catalytic activity, and superior electroconductibility. While SACs demonstrate high catalytic activity due to their unique electronic and geometric structures, they are prone to agglomeration under real-world operating conditions, which reduces their stability and catalytic performance. The rational design of diatomic catalysts (DACs) has emerged as an effective solution to address these issues. However, their application in aqueous zinc-iodine batteries (AZIBs) has yet to undergo experimental validation. Moreover, the catalytic mechanisms involved in iodine conversion with DACs remain unclear and require further investigation.
To address this gap, Song Yu's research group at NEU conducted an indepth study on the application of zinc-based diatomic catalysts in aqueous zinc-iodine batteries. They selected MnZn-NC as their model through computational screening, uncovering the key role of spin exchange interactions. The MnZn diatomic catalyst effectively suppresses polyiodide shuttle effects and promotes efficient electrochemical conversion of iodine. At a current density of 1Ag⁻¹, the MnZn-NC/I₂ cathode delivered a high specific capacity of 224mAhg⁻¹, surpassing the performance of both Zn-NC/I₂ and Mn-NC/I₂ single-atom catalysts. Additionally, the MnZn-NC system showed outstanding stability, with the zinc-iodine battery achieving over 320,000 cycles at 16Ag⁻¹, setting a new benchmark by extending the cycle life from 200,000 to beyond 300,000 cycles for the first time. Both experimental and theoretical results revealed that spin exchange interactions between the Mn-Zn atoms synergistically optimized their electronic structures, enhancing Mn's metallicity, raising Zn's d-band center, and strengthening the interaction between MnZn-NC and iodine species. This concept also extends to other bimetallic M-Zn-NC catalysts (M = Fe, Co, Ni, Cu), showcasing its versatility.
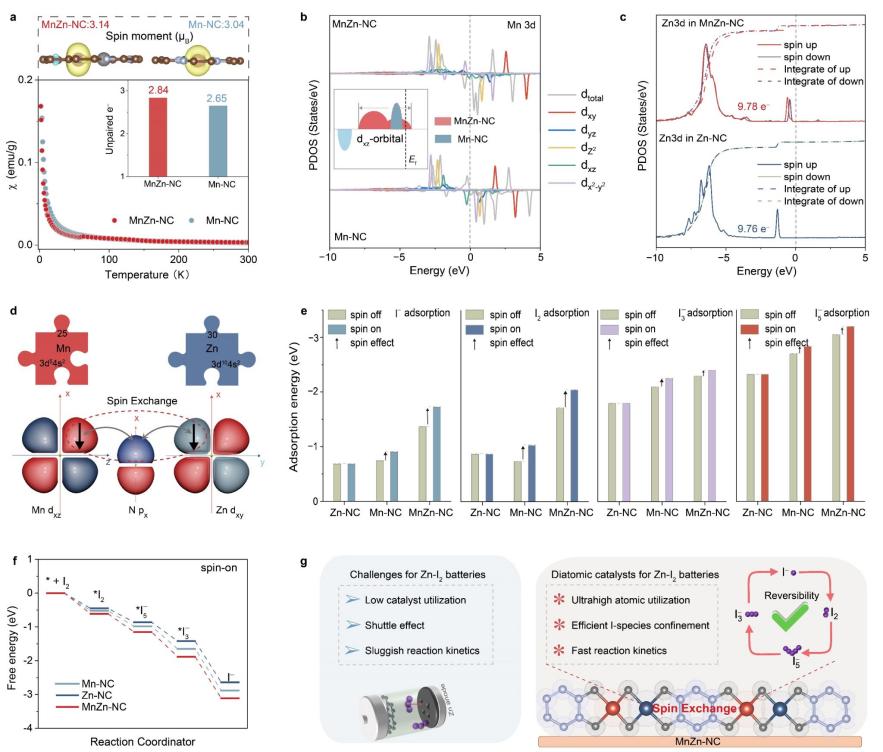
Figure 1: Theoretical research findings
The development of high-performance catalysts for iodine cathode reactions in AZIBs has long been a research hotspots in the scientific community. This study is the first to reveal the design principles and catalytic mechanisms of diatomic catalysts in advanced zinc-iodine batteries, providing a new idea for the development of other high-performance battery systems. NEU's Analysis and Testing Center provided critical support in material structure characterization, especially in morphological analysis and data collection.