Professor Jianhua Wang's team from NEU has made significant progress in the field of single-cell multidimensional analysis, and the related research has been published in the international journal ACS Nano under the title of "Multiplex Profiling of Biomarker and Drug Uptake in Single Cells Using Microfluidic Flow Cytometry and Mass Spectrometry". NEU is the first unit of the paper, with Professor Jianhua Wang, Professor Mingli Chen, and Professor Lim Chwee Teck from the NUS as co-corresponding authors, and Xuan Zhang, a PhD student of Class of 2018 from the College of Science, as the first author. (Link to original article: https://pubs.acs.org/doi/full/10.1021/acsnano.3c12803)
Tumor cells have significant single-cell heterogeneity and biomarker diversity, and multidimensional precision analysis at the single-cell level can help achieve precise diagnosis and personalized treatment of cancer. However, tumor cells are extremely rare in clinical samples, and traditional means of ultracentrifugation may cause sample damage and loss.
To address these issues, the team has developed a series of modular spectral-mass spectrometry two-dimensional single-cell detection platforms. The aptamer-labeled nano-fluorescent probes with high surface area can accurately identify tumor cells expressing specific proteins. Using the self-developed single-cell focusing alignment—sample purification—fluorescence/mass spectrometry sequential detection micro-total analysis (μTAS) chip, we can focus, align, and purify the target cells in complex biological samples, and realize the single-cell high-resolution and high-sensitivity recognition and detection of single cells in two-dimensional mode by laser-induced fluorescence (LIF) and inductively coupled plasma mass spectrometry (ICPMS). This system allows assessment of the association between cellular uptake of platinum-based drugs with induction of PTK7 protein expression at the individual cell level, thereby achieving the phenotypic differentiation of rare tumor cells.
The enhanced helical single-cell focused microfluidic chip enables high-throughput alignment of single cells at gentle flow rates, eliminating the redundant centrifugal cleaning step in traditional cell sample processing. It greatly saved sample purification time and avoided unnecessary sample loss; the large surface area of the nanoparticles coupled with nearly 3,000 Sgc8 aptamers can form a high-affinity aptamer nanoprobe, which effectively recognizes all target cells in the sample. This scheme provides a new idea for the detection and phenotypic analysis of rare tumor cells in whole blood samples.
It is reported that the relevant research is based on the multidimensional detection platform independently developed by the National Major Scientific Instruments and Equipments Development Project of Natural Science Foundation of China (completed, NSFC 21727811), and the research content is funded by the Key Project of National Natural Science Foundation of China (NSFC22334003) and the Start-Up Grant of NUS (A-8001301-00-00).
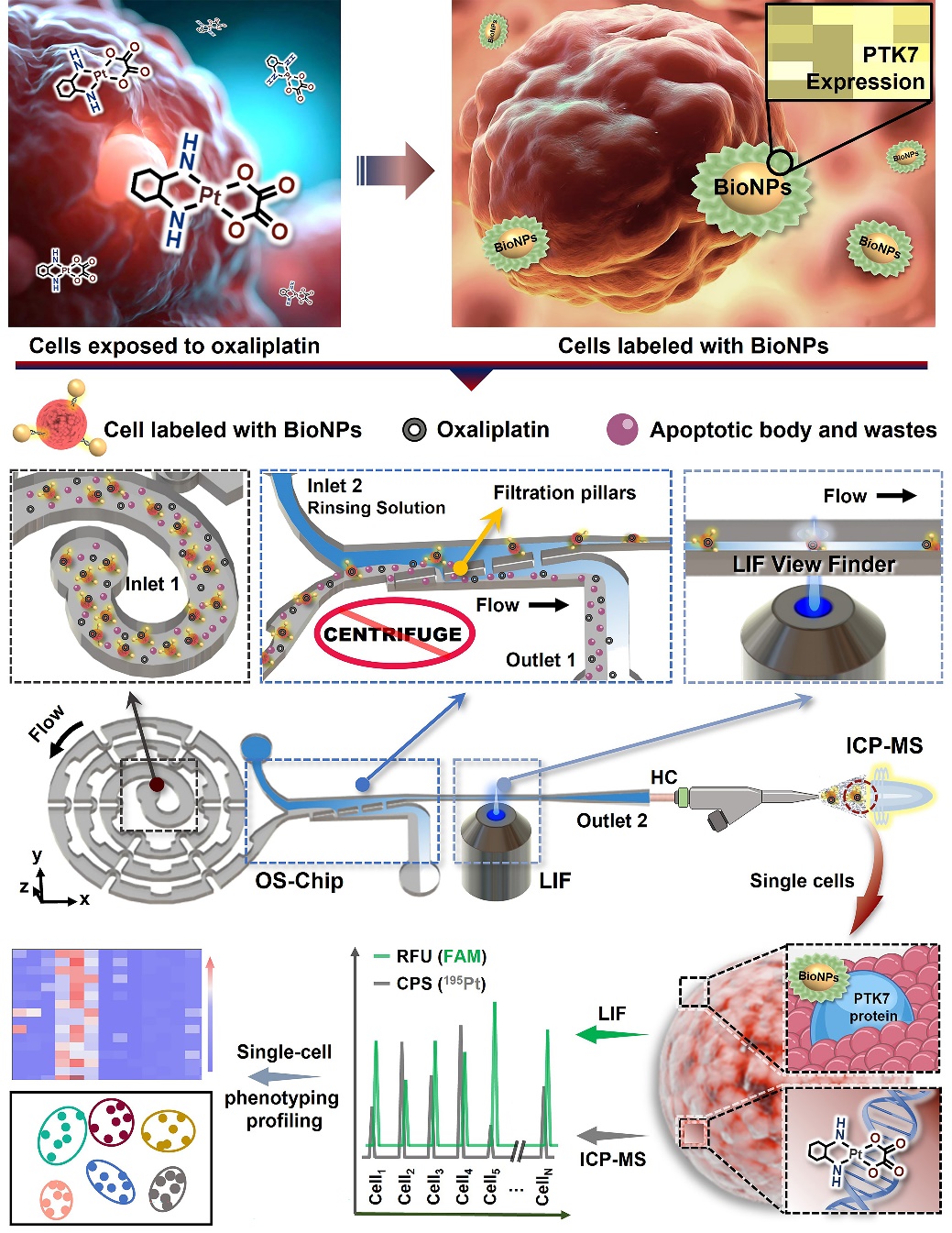
Schematic diagram of single-cell spectral/mass spectrometry phenotypic analysis